Fluoroalkylation of Various Nucleophiles with Fluoroalkyl Sulfones Through a Single Electron Transfer Process 
Xiao, Pan; Ni, Chuanfa; Miao, Wenjun; Zhou, Min; Hu, Jingyu; Chen, Dingben; Hu, Jinbo*
Journal of Organic Chemistry 2019, DOI: 10.1021/acs.joc.9b00419.
(Selected as "Featured Article")
Abstract:
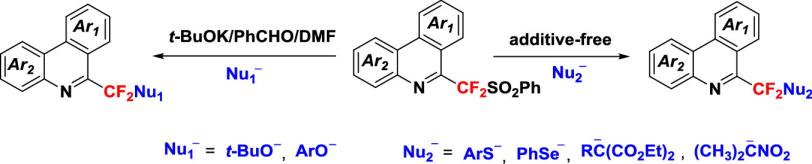
The fluoroalkylation of various nucleophilic reagents with (phenylsulfonyl)difluoromethyl (PhSO2CF2)-substituted phenanthridines was achieved to obtain fluorinated phenanthridine derivatives, which enables the construction of both carbon—heteroatom and carbon—carbon bonds via nucleophilic substitution of the phenylsulfonyl group. Mechanistic studies indicated that these reactions proceed through unimolecular radical nucleophilic substitution (SRN1) mechanism. It is worthwhile noting that in the cases of O-nucleophiles (t-BuO- and PhO-), the further addition of t-BuOK/PhCHO could significantly promote the reactions, due to the in-situ formation of a highly reactive electron donor species through the interaction of t-BuOK, PhCHO and the solvent DMF, which can effectively initiate the SET process.

